#homebrew #BrewBoss
As many of you know, the #alphaacids found in #hops are what is responsible for the hop flavor and bitterness in beer. Alpha acids, usually reported as a percent, is the variable most people use to calculate the #IBU of the beer they are making. It isn’t just one acid, the term “alpha acid” refers to a group of acids found in hop flowers that include humulone, adhumulone, cohumulone, posthumulone, and prehumulone. When hops are added to boiling wort, an isomerization happens in the boil and the alpha acids are converted to iso-alpha acids. Since the most common alpha acid is humulone, the most common iso-alpha acids are cis- and trans-isohumulone.
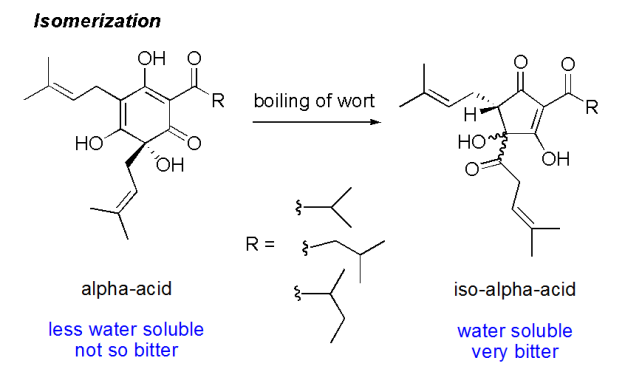
Isomerization of alpha acid humulone to iso-humulone
There are several ways to mathematically estimate what the IBUs of a beer are going to be based on the rough alpha acid concentration reported on the bag of hops you use. The three most popular calculations are the Rager, Tinseth, and Garetz equations. I personally use the Tinseth equation.

Tinseth Equation
I like the Tinseth equation because it takes into account the gravity of wort, which affects solubility of the acids. You can read all about that sort of thing on Greg’s page. Other calculations often ignore this factor, which could lead to significant differences in expected and resulting concentrations of alpha acid. I believe many online calculators use the Rager equation which also takes into consideration gravity of the wort.

Rager Equation
So while researching how to measure IBU, I found an old protocol from the 1960s on how to measured the concentration of iso-alpha acids (and therefore IBUs) of a beer. First, I ordered some 2,2,4-Trimethylpentane also known as isooctane.

Trimethylpentane
This method of measuring the amount of isomerized alpha acid in solution is not the current method used (I’ll write about that one later) but it is an older method that is easy to do. The principle here is to do an organic extraction of the beer. By mixing the isooctane with the beer, the two naturally separate, like oil and water. The isooctane “floats” on top of the beer but by vortexing the solution for seveal minutes, the organic components of the beer can be moved to the organic phase, the isomerized acids are soluble in the isooctane and get absorbed into that phase. Then the task becomes measuring how much alpha acid is in the organic phase. This was done by doing a spectra of the solution and measuring absorbance at 275 nm, a wavelength of light absrobed by the iso-humulone. Knowing that number and the extinction coefficent of iso-humulone, calculating the IBU was easy after there.
Protocol
- In a 15 ml conical tube mix
- 1 ml of beer (degassed)
- 100 μl of 3 M HCl
- 2 ml of iso-octane
Vortex for 2 minutes to make an emulsion of the organic and aqueous phases. This shaking allows for the extraction of the acid into the organic phase. Allow the phases to separate and remove organic phase. Read absorbance at 275 nanometers against reference of iso-octane.
Calculation is EBU (European bittering Units) = 50 * A275
I cannot locate it now, but the paper I was using for this protocol had a table that converted EBU to IBU. I tried to Google it but there seemed to be a disagreement online if there is a difference. The paper I was using was from the 60s so there was likely a correction added for the coversion from metric to English units. That is the likely difference although the numbers were really close. Once I find it, I will take a photo of the table and post it on here (hopefully tonight).
UPDATE: I found it.

Table comparing IBU and EBU
I decided to test three samples, two Galaxy saisons I’ve brewed recently and a homebrew from a friend that we both agreed was really bitter.
Homebrewed examples
Beer Calculated IBU A275 Measured IBU (EBU)
Galaxy Saison Dupont 40 0.946 39.6 (47.3)
Galaxy Saison Blaugies 35 0.900 37.4 (45)
Niciu IPA unknown 4.31 (crazy bitter beer) >150 (215)
The Niciu IPA, provided by a friend and fellow homebrewer, was made for an “unreasonably hopped” club competition for the DC Homebrewers. The original extraction reading was “out of range” for the instrument I was using, meaning it was higher than “3”. I made a 1 in 10 dilution of the organic and got a reading of 0.431….this is likely not an accurate reading for a few reasons but this is a crazy bitter beer, so I marked it in the table as >150, apparently it is out of the range I can detect.
It shouldn’t surprise me that my numbers were close to the deconvulated IBU numbers given by the table in the paper…I was surprised how close. I’m also quite shocked with the result of the Niciu IPA. I think one of the problems with this technique is the linear range and reproducibility. I’m going to test a lot more samples in the coming months but overall this is a relatively silly technique. It requires pipetting the same amount of isooctane into several samples, there are several variables that might affect the outcome of the extraction, such as temperature and time mixing. I tried to keep these things as similar as possible but even with just three samples I ended up with variation in the organic phase, the Niciu IPA had a precipitate on top of the organic phase. Obviously if this were a lab, the samples would be tested multiple times to get enough data to do some stats but I’m not going to bother.
I hope people found this interesting, I loved doing it. Hopefully I can research additional techniques for measuring hops contributions to beer and write about those as a follow-up.
“Some people wanted champagne and caviar when they should have had beer and hot dogs.” –Dwight D. EisenhowerSource:
http://phdinbeer.com/2014/09/16/beer-chemistry-1-measuring-ibus-in-beer/
No comments:
Post a Comment